Discovery and Study of Modified Genes
Domesticated genes can be identified and isolated by using technologies as quantitative trait locus (QTL) analysis, genome-wide association studies (GWAS) and whole genome resequencing studies. GWAS identifies significant associations between genes and phenotypic traits by using markers, focusing on single-nucleotide polymorphisms (SNPs) in the genome and linkage disequilibrium to provide full coverage (Meyer and Purugganan, 2013).
Quantitative Trait Locus (QTL) mapping (or Linkage mapping)
is a technique that is among the first used in the study of domesticated traits (Doebley et al., 2006; Gross and Olsen, 2010). According to Meyer and Purugganan (2013), QTL is defined as a “genomic region with a gene (or multiple linked genes) that contains mutations which result in phenotypic variation in populations” (Meyer and Purugganan, 2013). And the main goal of the QTL mapping is to help study less understood phenotypes by characterizing (number, location, and impact) the determinants involved in the inheritance of variable traits (Paterson, 2002). The methodology consists in, starting with the crossing of F0 strains genetically and phenotypically different in the trait of interest (the wild-type and the domesticated). Then develop a mapping population of the F1 genetically marked generation (using techniques such as double haploid lines, backcross, or recombinant inbred lines for example), followed by the construction of a linkage map that characterize the segregation of markers in the chromosomes, and finally a statistical study to relate the genetic and phenotypic variance (Kearsey, 1998; Miles and Wayne, 2008; Sehgal et al., 2016). First, we map the markers and then we relate the markers and the trait (Kearsey and Farquhar, 1998). Some of the problems associated with this technique are the small individual QTL effects; don’t work well with certain crops, like perennial’s for example; some statistical difficulties; lack of specificity; and the need of a phenotype identification beforehand (Kearsey, 1998; Doebley et al., 2006; Ross-Ibarra et al., 2007).
Linkage Disequilibrium (LD), or Association, mapping
Mackay and Powell (2007) define linkage disequilibrium as “the non-random association of alleles at separate loci located on the same chromosome”, and this process can be caused by several factors such as mutations, migrations, and selection for example (Mackay and Powell, 2007). This technique, first used in human genetics, can be made in various ways (Multiparent Advanced Generation Intercross or Transmission Disequilibrium Test), but, they all have the same basis, by relying in the LD decay, and comparing genome variation with phenotype variations and the more in-dept analysis of the responsible genetic alterations, making use of molecular markers and statistical analysis (Mackay and Powell, 2007; Ross-Ibarra et al., 2007; Sehgal et al., 2016). Although more rapid and with better mapping resolution than QTL mapping, due to the lack of necessity of doing crossovers, there is a need for a large sample size and some problems associated with the geographic origin of the studied individuals, that can affect the veracity of the results (Ross-Ibarra et al., 2007; Sorkheh et al., 2008; Sehgal et al., 2016).
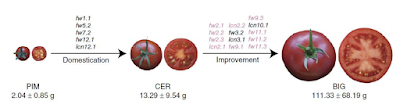
Made by : Celso Santos and Patrícia Cruz
Made by : Celso Santos and Patrícia Cruz
Bibliography
Bai, Y. and Lindhout, P. (2007). Domestication and Breeding of Tomatoes: What have We Gained and What Can We Gain in the Future? Annals of Botany, 100(5), pp.1085-1094.
Baldwin, E.A. (1993). Citrus fruit. In Biochemistry of Fruit Ripening, pp. 107–149.
Bergougnoux, V. (2014). The history of tomato: From domestication to biopharming. Biotechnology Advances, 32(1), pp.170-189.
Blanca, J., Montero-Pau, J., Sauvage, C., Bauchet, G., Illa, E., Díez, M., Francis, D., Causse, M., van der Knaap, E. and Cañizares, J. (2015). Genomic variation in tomato, from wild ancestors to contemporary breeding accessions. BMC Genomics, 16(1).
Brewer, M., Moyseenko, J., Monforte, A. and van der Knaap, E. (2007). Morphological variation in tomato: a comprehensive study of quantitative trait loci controlling fruit shape and development. Journal of Experimental Botany, 58(6), pp.1339-1349.
Charmet, G. (2011). Wheat domestication: Lessons for the future. Comptes Rendus Biologies, 334(3), pp.212-220.
Cong, B., Liu, J. and Tanksley, S. (2002). Natural alleles at a tomato fruit size quantitative trait locus differ by heterochronic regulatory mutations. Proceedings of the National Academy of Sciences, 99(21), pp.13606-13611.
Diamond, J. (2002). Evolution, consequences and future of plant and animal domestication. Nature, 418(6898), pp.700-707.
Doebley, J., Gaut, B. and Smith, B. (2006). The Molecular Genetics of Crop Domestication. Cell, 127(7), pp.1309-1321.
Eckardt, N. (2010). Evolution of Domesticated Bread Wheat. The Plant Cell, 22(4), pp.993-993.
Faris, J. (2014). Wheat Domestication: Key to Agricultural Revolutions Past and Future. In: Tuberosa, R., Graner, A. and Frison, E. (n.d.). Genomics of plant genetic resources.
Faris, J., Zhang, Z. and Chao, S. (2014). Map-based analysis of the tenacious glume gene Tg-B1 of wild emmer and its role in wheat domestication. Gene, 542(2), pp.198-208.
Farrell, B., Sequeira, A., O'Meara, B., Normark, B., Chung, J. and Jordal, B. (2001). The evolution of Agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution, 55(10), p.2011.
Frary, A. (2000). fw2.2: A Quantitative Trait Locus Key to the Evolution of Tomato Fruit Size. Science, 289(5476), pp.85-88.
Fuller, D. (2007). Contrasting Patterns in Crop Domestication and Domestication Rates: Recent Archaeobotanical Insights from the Old World. Annals of Botany, 100(5), pp.903-924.
Gross, B. and Olsen, K. (2010). Genetic perspectives on crop domestication. Trends in Plant Science, 15(9), pp.529-537.
Hummer, K., Bassil, N. and Njuguna, W. (2010). Fragaria. Wild Crop Relatives: Genomic and Breeding Resources, pp.17-44.
Kearsey, M. and Farquhar, A. (1998). QTL analysis in plants; where are we now? Heredity, 80(2), pp.137-142.
Kearsey, M.J. (1998). The principles of QTL analysis (a minimal mathematics approach). Journal of Experimental Botany, 49(327), pp.1619–1623.
Keller, S. and Taylor, D. (2010). Genomic admixture increases fitness during a biological invasion. Journal of Evolutionary Biology, 23(8), pp.1720-1731.
Koenig, D., Jimenez-Gomez, J., Kimura, S., Fulop, D., Chitwood, D., Headland, L., Kumar, R., Covington, M., Devisetty, U., Tat, A., Tohge, T., Bolger, A., Schneeberger, K., Ossowski, S., Lanz, C., Xiong, G., Taylor-Teeples, M., Brady, S., Pauly, M., Weigel, D., Usadel, B., Fernie, A., Peng, J., Sinha, N. and Maloof, J. (2013). Comparative transcriptomics reveals patterns of selection in domesticated and wild tomato. Proceedings of the National Academy of Sciences, 110(28), pp.E2655-E2662.
Konishi, S., Izawa, T., Lin, S., Ebana, K., Fukuta, Y., Sasaki, T. and Yano, M. (2006). An SNP Caused Loss of Seed Shattering During Rice Domestication. Science, 312(5778), pp.1392-1396.
Kovach, M., Sweeney, M. and McCouch, S. (2007). New insights into the history of rice domestication. Trends in Genetics, 23(11), pp.578-587.
Lai, X., Hinga, M., Lobos, K., Martinez, C., McCouch, S., Thomson, M., Tai, T., McClung, A. and Xu, Y. (2003). Mapping quantitative trait loci for yield, yield components and morphological traits in an advanced backcross population between Oryza rufipogon and the Oryza sativa cultivar Jefferson. TAG Theoretical and Applied Genetics, 107(3), pp.479-493.
Lin, T., Zhu, G., Zhang, J., Xu, X., Yu, Q., Zheng, Z., Zhang, Z., Lun, Y., Li, S., Wang, X., Huang, Z., Li, J., Zhang, C., Wang, T., Zhang, Y., Wang, A., Zhang, Y., Lin, K., Li, C., Xiong, G., Xue, Y., Mazzucato, A., Causse, M., Fei, Z., Giovannoni, J., Chetelat, R., Zamir, D., Städler, T., Li, J., Ye, Z., Du, Y. and Huang, S. (2014). Genomic analyses provide insights into the history of tomato breeding. Nature Genetics, 46(11), pp.1220-1226.
Mackay, I. and Powell, W. (2007). Methods for linkage disequilibrium mapping in crops. Trends in Plant Science, 12(2), pp.57-63.
Martínez-Ainsworth, N. and Tenaillon, M. (2016). Superheroes and masterminds of plant domestication. Comptes Rendus Biologies, 339(7-8), pp.268-273.
Meyer, R. and Purugganan, M. (2013). Evolution of crop species: genetics of domestication and diversification. Nature Reviews Genetics, 14(12), pp.840-852.
Miles, C. and Wayne, M. (2008) Quantitative trait locus (QTL) analysis. Nature Education 1(1):208.
Paran, I. and van der Knaap, E. (2007). Genetic and molecular regulation of fruit and plant domestication traits in tomato and pepper. Journal of Experimental Botany, 58(14), pp.3841-3852.
Paterson, A. (2002). What has QTL mapping taught us about plant domestication? New Phytologist, 154(3), pp.591-608.
Peleg, Z., Fahima, T., Korol, A., Abbo, S. and Saranga, Y. (2011). Genetic analysis of wheat domestication and evolution under domestication. Journal of Experimental Botany, 62(14), pp.5051-5061.
Poncet, V., Robert, T., Sarr, A., and Gepts, P. (2004). Quantitative trait loci analyses of the domestication syndrome and domestication process. Encyclopedia of Plant and Crop Science, 1069–1073.
Purugganan, M. and Fuller, D. (2009). The nature of selection during plant domestication. Nature, 457(7231), pp.843-848.
Ross-Ibarra, J., Morrell, P. and Gaut, B. (2007). Plant domestication, a unique opportunity to identify the genetic basis of adaptation. Proceedings of the National Academy of Sciences, 104(Supplement 1), pp.8641-8648.
Rousseau-Gueutin, M., Gaston, A., Aïnouche, A., Aïnouche, M., Olbricht, K., Staudt, G., Richard, L. and Denoyes-Rothan, B. (2009). Tracking the evolutionary history of polyploidy in Fragaria L. (strawberry): New insights from phylogenetic analyses of low-copy nuclear genes. Molecular Phylogenetics and Evolution, 51(3), pp.515-530.
Rousseau-Gueutin, M., Lerceteau-Kohler, E., Barrot, L., Sargent, D., Monfort, A., Simpson, D., Arus, P., Guerin, G. and Denoyes-Rothan, B. (2008). Comparative Genetic Mapping Between Octoploid and Diploid Fragaria Species Reveals a High Level of Colinearity Between Their Genomes and the Essentially Disomic Behavior of the Cultivated Octoploid Strawberry. Genetics, 179(4), pp.2045-2060.
Schaffner, S. and Sabeti, P. (2008) Evolutionary adaptation in the human lineage. Nature Education, 1(1):14.
Schultz, T. and Brady, S. (2008). Major evolutionary transitions in ant agriculture. Proceedings of the National Academy of Sciences, 105(14), pp.5435-5440.
Sehgal D., Singh R., Rajpal V.R. (2016) Quantitative Trait Loci Mapping in Plants: Concepts and Approaches. In: Rajpal V., Rao S., Raina S. (eds) Molecular Breeding for Sustainable Crop Improvement. Sustainable Development and Biodiversity, vol 11. Springer, Cham.
Simons, K. J., Fellers, J. P., Trick, H. N., Zhang, Z., Tai, Y.-S., Gill, B. S., & Faris, J. D. (2006). Molecular Characterization of the Major Wheat Domestication Gene Q. Genetics, 172(1), 547–555.
Sorkheh, K., Malysheva-Otto, L., Wirthensohn, M., Tarkesh-Esfahani, S. and Martínez-Gómez, P. (2008). Linkage disequilibrium, genetic association mapping and gene localization in crop plants. Genetics and Molecular Biology, 31(4), pp.805-814.
Tanksley, S. (2004). The Genetic, Developmental, and Molecular Bases of Fruit Size and Shape Variation in Tomato. THE PLANT CELL ONLINE, 16(suppl_1), pp.S181-S189.
Van der Hoeven, R. (2002). Deductions about the Number, Organization, and Evolution of Genes in the Tomato Genome Based on Analysis of a Large Expressed Sequence Tag Collection and Selective Genomic Sequencing. THE PLANT CELL ONLINE, 14(7), pp.1441-1456.
Wu, G., Terol, J., Ibanez, V., López-García, A., Pérez-Román, E., Borredá, C., Domingo, C., Tadeo, F., Carbonell-Caballero, J., Alonso, R., Curk, F., Du, D., Ollitrault, P., Roose, M., Dopazo, J., Gmitter, F., Rokhsar, D. and Talon, M. (2018). Genomics of the origin and evolution of Citrus. Nature, 554(7692), pp.311-316.

Comentários
Postar um comentário